Intranasal Naloxone
(NS-001)
Access to naloxone is a priority for reducing opioid deaths. Intranasal Naloxone aimed at assisting in acute emergency situations like opioid overdose.

Medical need
During 2022 over 100000 opioid related death occurred in the US
Regulatory requirements
Bioequivalence study –”equal or better”
Nasus pharma presented clinical results at Fourth Annual
ASPN Conference, July 14-17, Miami Beach
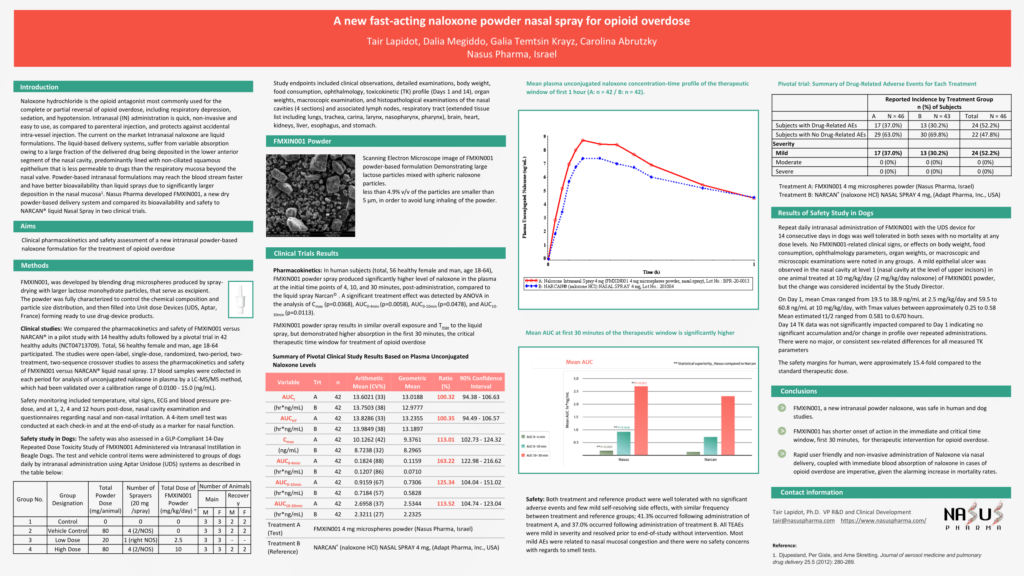
Nasus Pharma announces publication of its pivotal study comparing its FMXIN001 powder nasal Naloxone to Narcan® – READ MORE
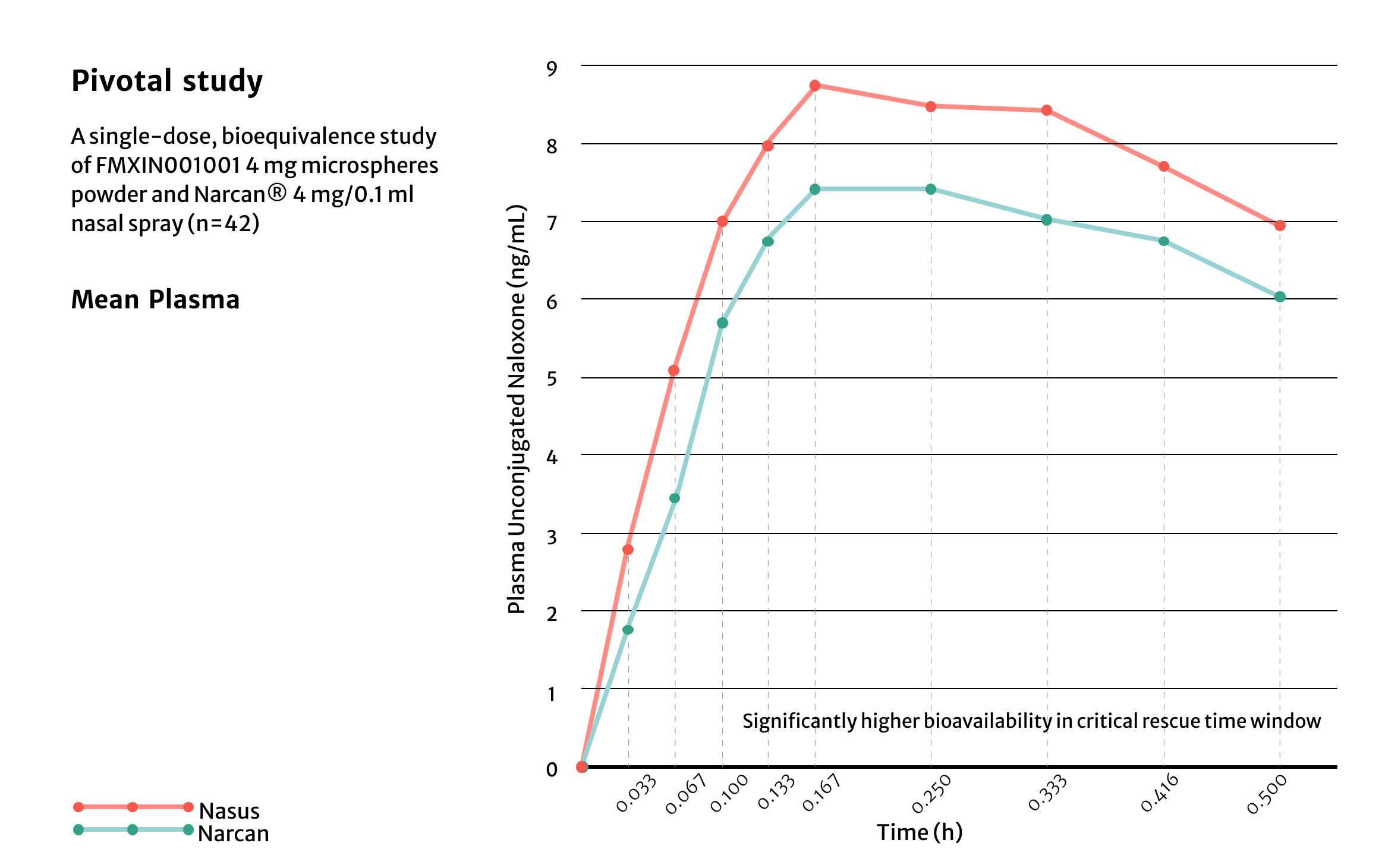
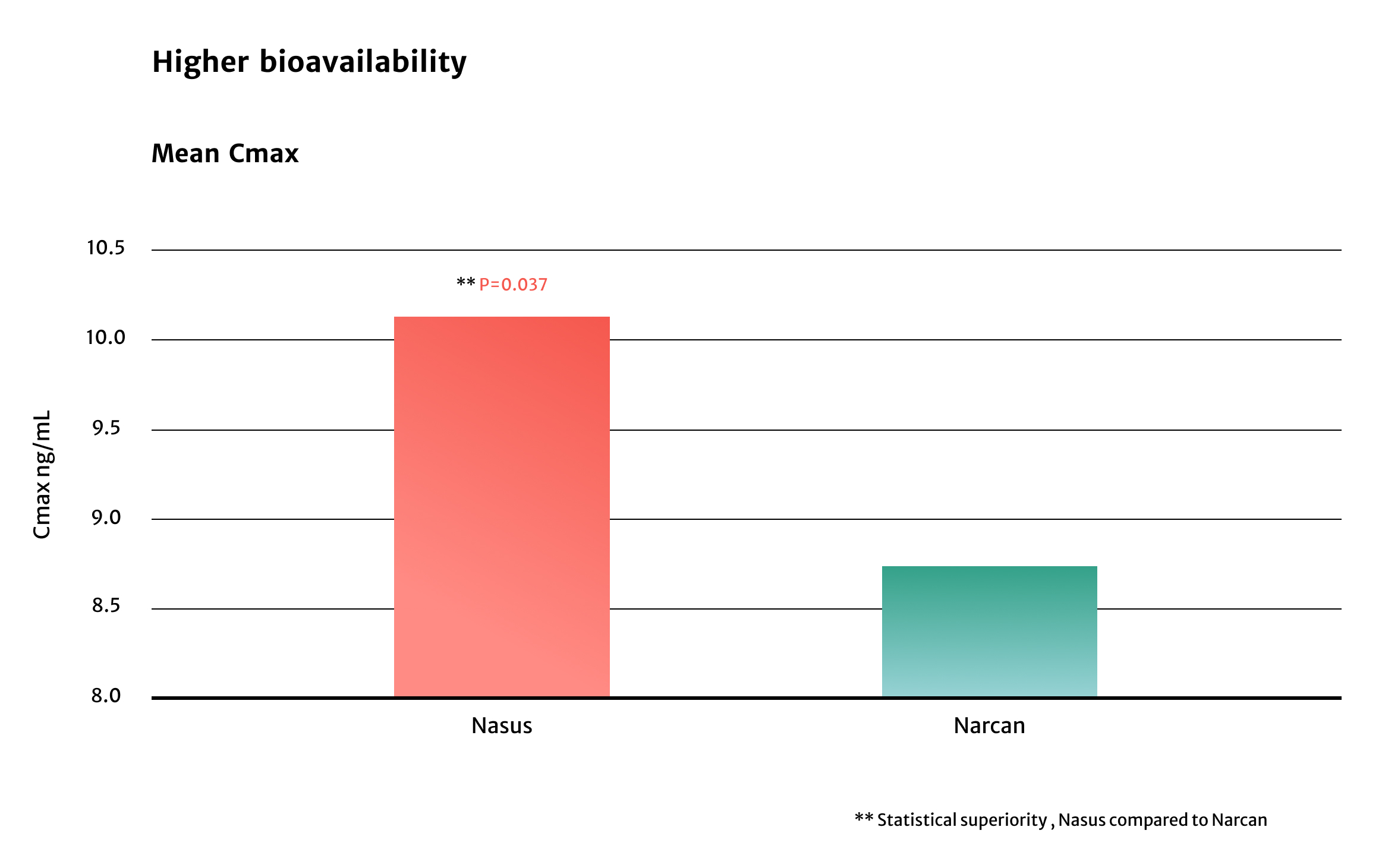
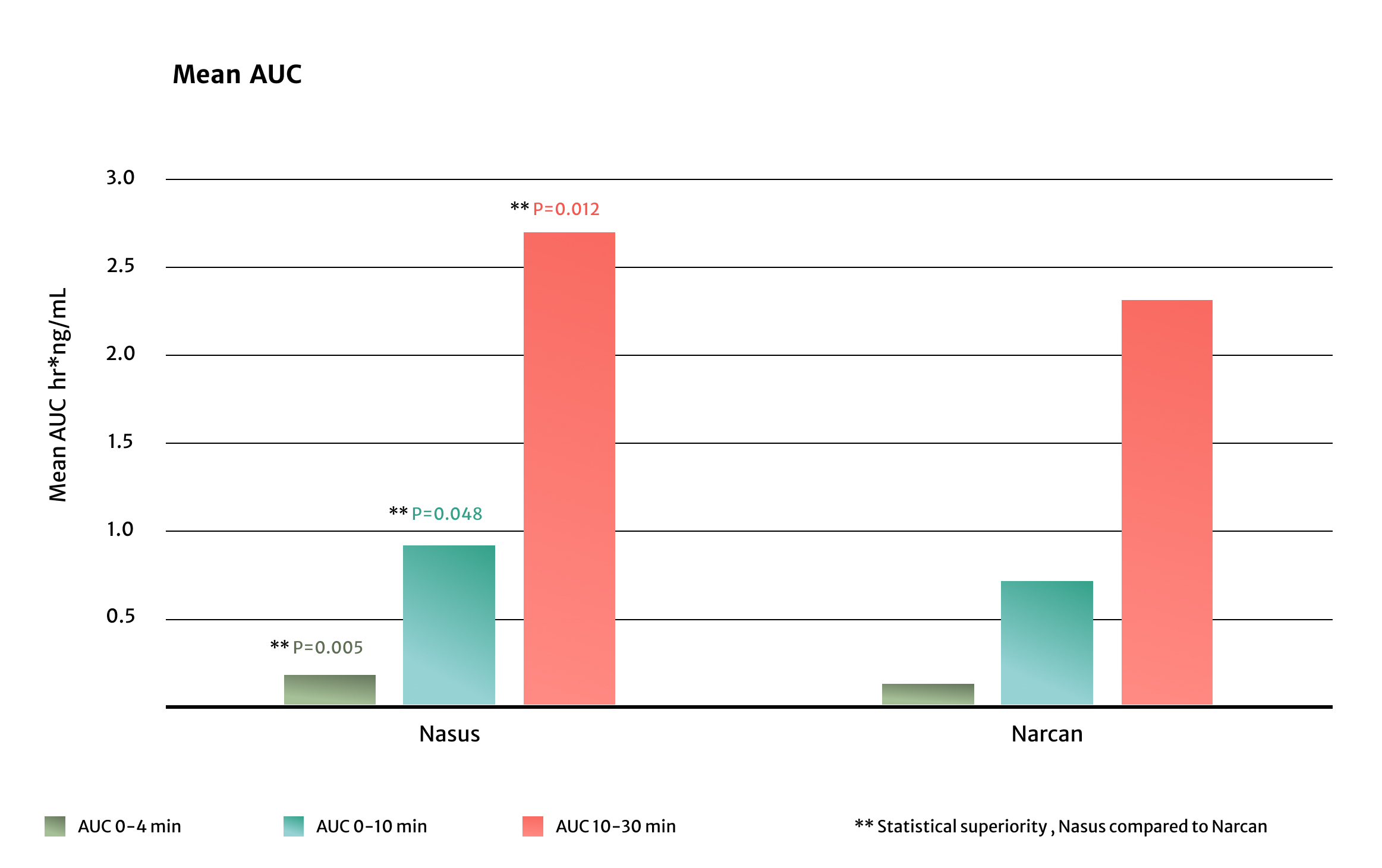
Pilot Study
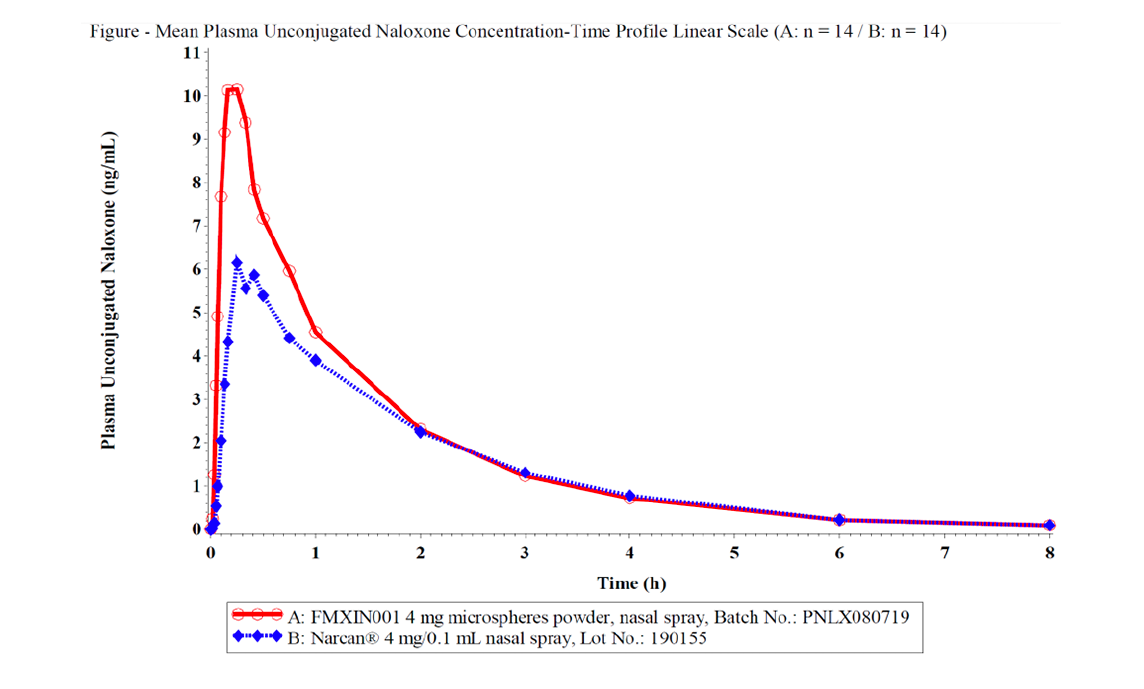
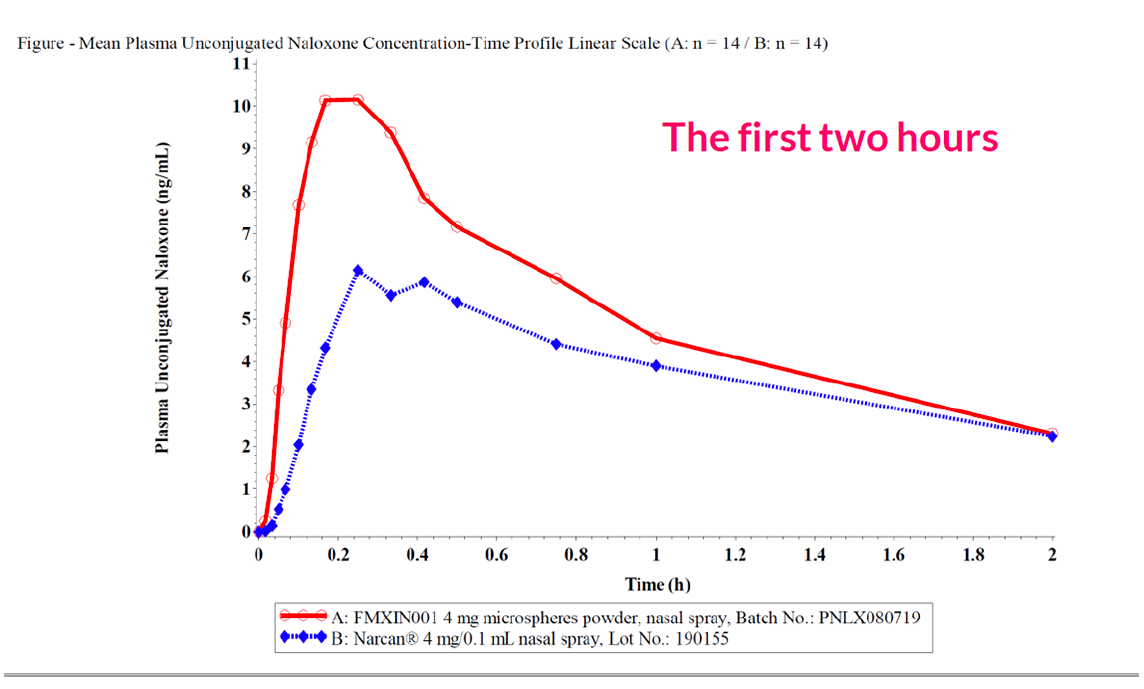
The peak systemic exposure was approximately
1.6 fold higher
The partial AUC0-4 minutes was approximately
7 fold higher
The partial AUC0-10 minutes was approximate
4 fold higher
The partial AUC10-30 minutes was approximate
1.6 fold higher
The total systemic exposure was approximate
1.26 fold higher
The median Tmax occurred approximately
5 minutes earlier